Abstract
Microscopic examination of red blood cell (RBC) morphology in a peripheral blood smear is a standard clinical test with broad utility even in resource-limited settings. Yet this analysis remains subjective, semi-quantitative, and low throughput, limiting its potential in clinical practice and translational research. Prior attempts to develop quantitative RBC morphology tools have been hampered by limited scope, reproducibility challenges, and sparse clinical validation.
Here, we present a novel, open-source machine-learning approach leveraging durable geometric features to quantify clinically relevant RBC morphologies-elliptocytes, microcytes, macrocytes, schistocytes, sickle cells, spiculated cells, and teardrop cells-and generate an RBC differential ('RBC-diff'), analogous to the widely used white cell differential. Analysis of >300 thousand smear images across two medical centers shows algorithm concordance with expert human morphology estimates at both the individual cell and full-field smear level, and when compared to standard clinical grading of smears. This algorithm illustrates high intra-sample reproducibility while providing greatly improved speed and resolution comparative to current standards.
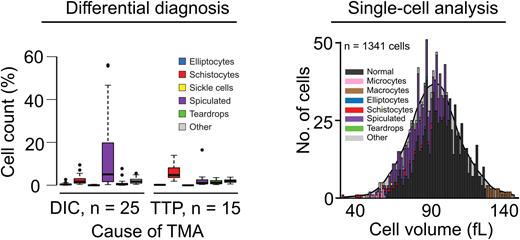
To test the utility of this new tool we examined: (i) diagnostic signatures in the RBC differential, (ii) large-scale cohort prognostics, (iii) single-cell mechanistic studies, and (iv) an animal model of hematologic disease. Diagnostic RBC differential signatures were discovered by applying the RBC-diff to a cohort of patients with thrombotic microangiopathy (TMA). In particular, the RBC-diff facilitated rapid identification and differential diagnosis of patients with thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS), providing greater specificity than manual review (72% vs. 41%, p < 0.001), while maintaining high sensitivity (94-100%). Because TTP is a life-threatening disorder requiring immediate therapy, this new rapid diagnostic tool may provide significant benefit, as existing gold-standard diagnostic tests for TTP are slow (hours to days). Large-scale cohort prognostic value was uncovered through an analysis of smears from a general cohort of 58,950 inpatients that found that elevated schistocyte counts are associated with increased all-cause mortality (9.5% six-month mortality for >1% schistocytes vs. 4.7% for schistocytes < 0.5%, p < 0.001), after adjusting for patient demographics, comorbidities, and manual smear review flags. This tool also allowed for analysis of morphologically annotated single-cell volume distributions, providing insights into how dysmorphology can affect mean corpuscular volume (MCV) and the red cell distribution width (RDW); note that current technology provides only whole-population measures for these variables. For example, in iron-deficient patients who underwent intravenous iron transfusions, this technique highlighted that successful hematologic resolution involved volume increases for all cell types, rather than an isolated reduction in microcyte levels. Finally, without any retraining the model was successfully applied to animal-derived blood smears, highlighting expected differences between a wild-type (WT) and sickle cell disease mouse model (SCD) (0% sickle cells for WT, 1.2% for SCD), demonstrating potential value for basic research investigations.
Usage of the red cell differential has the potential to greatly benefit hematologic research and clinical practice. Here we present the RBC-diff, an extensively validated method for efficient calculation of this differential from commonly collected blood smear images. As a resource to the community, we provide open-source code for calculating the RBC-diff. We also provide a repository of 5000 individual RBC images, and 50 full-field smears, each manually labelled by a team of expert pathologists, to facilitate further developments of improved computational methods of RBC classification.
Disclosures
Bendapudi:Alexion: Consultancy; Takeda: Consultancy. Al-Samkari:argenx: Consultancy; Rigel: Consultancy; Novartis: Consultancy; Amgen: Research Funding; Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Forma: Consultancy; Moderna: Consultancy; Dova: Consultancy, Research Funding. Louissaint:Lymphoma Research Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal